Most research in our lab focuses on the nitrogen (N) cycle.
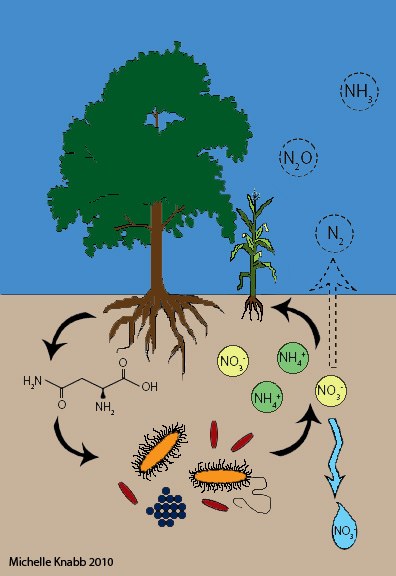
Illustration of the Nitrogen cycle in soil, plants, and air
People have been interested in N for years because a lack of N often limits plant growth. More recently, with increased synthetic fertilizer use, excess soil N has become a concern.
Fertilizers are often added to soil as inorganic N, such as nitrate (NO3) and ammonium (NH4) shown here in yellow and green circles. These are readily used by plants, but other processes compete with plants for inorganic N. NO3 is leached away as water moves through soil (light blue drop), some soil organisms convert NO3 to the greenhouse gas nitrous oxide (N2O), and NH4 can be lost as ammonia (NH3) gas.
When leaves or roots senesce, plant N is returned to soil in organic forms, such as amino acids (black molecule at left).
When organic N is abundant, soil microorganisms (orange, red, and blue creatures at the center-bottom) convert it to inorganic N again (a process called mineralization). In contrast, when organic N is in short supply relative to carbon, soil organisms will compete with plants for inorganic N.
Some very important processes are not depicted in this cartoon (we had to stop somewhere). Many plants, including important agricultural crops, form symbioses with soil microorganisms that take N2 gas out of the atmosphere and convert it to forms that are useful for growth (called N fixation). In some cases, plants will take up simple amino acids from soil, so the arrow between plants and the amino acid could go both ways. We show organic N as a free amino acid molecule, but organic N actually exists in many forms in soil and can be dissolved in soil solution or associated with soil minerals. Soil minerals also have cation exchange sites that attract NH4, limiting its movement in soil. With sufficient clay, the components shown here (organic compounds, inorganic N, and microorganisms) can be closely associated and protected in soil aggregates. Finally, in many agricultural ecosystems, animal waste is a major input of both organic N and NH4 to soil that is not explicit in our cartoon.
One of the greatest challenges of the future will be to find ways to provide enough N to crops to maintain high yields while minimizing nutrient pollution that occurs when N is lost as gases or leached.
The goals of our research are to:
- increase basic understanding of N cycling in plants and soils
- help minimize N losses from managed ecosystems
- discover how N losses from managed ecosystems affect and are attenuated by unmanaged ecosystems.
We measure the flow and fate of N at scales ranging from individual microbial processes, to whole ecosystems and landscapes, to regions.
The Kaye Biogeochemistry Lab believes that everyone should have equal access to science, and we strive to create an environment that welcomes and respects diversity in all its forms—including cultural, racial, religious, age, gender identity, sexual orientation, physical ability, and mental wellbeing. Read more here.
The Kaye Biogeochemistry Lab believes that everyone should have equal access to science, and we strive to create an environment that welcomes and respects diversity in all its forms—including cultural, racial, religious, age, gender identity, sexual orientation, physical ability, and mental wellbeing. Read more here.

