UNICUBE Elemental CHNS-O Organic Analyzer
Principle: This analysis is based on flash combustion of the sample transforming solids into the gas phase as a result of instantaneous oxidation under O2 flow. The combustion products are separated by a chromatographic adsorption column which utilizes temperature programmed desorption where each gas evolves at a particular temperature. The detection is performed by a thermal conductivity detector (TCD) that compares the thermal conductivity of a reference gas to the one evolving from sample. TCD gives an output signal proportional to the concentration of the individual components in a material.
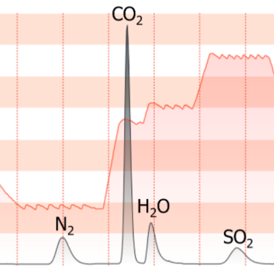
Capabilities: UNICUBE simultaneously determines the percentages of C, H, N , and S of organic compounds. UNICUBE also has a separate module to measure oxygen.
Detection limits (suggested) : C- 40 ppm, N – 100 ppm, H – 0 ppm, S – 2 ppm, O – 100 ppm.
Sample amount required for the analysis: about 10-15 g of plant material, 50-60 mg of soils; ground powder is recommended

Rigaku NEX CG II X-ray Fluorescence spectrometer
Principle: X-ray Fluorescence (XRF) spectroscopy measures the interaction of X-ray with the sample. When X-ray strike matter, some of them are adsorbed and some pass through. Adsorption and penetration depend on the elemental composition, density, and thickness of the sample. The consequence of adsorption is that secondary X-rays (fluorescent light) are generated which are characteristic of that matter. The energies of the fluorescent light correspond to the atomic structure and thus to the element type. XRF peaks with varying intensities are created and will be present in the spectrum, a graphical representation of X-ray intensity peaks as a function of energy peaks. The peak energy identifies the element, and the peak height/intensity is generally indicative of its concentration.

Capabilities: Non-destructive elemental analysis for sodium (Na) to uranium (U) of solids, liquids, powders, coatings, and thin films. High-power 50 kV, 50 W palladium-anode X-ray tube and Large-area high-throughput silicon drift detector (SDD)
Detection limits: low ppm to few percent(element and matrix dependent)
Sample amount required for the analysis: about 5 to 10 g of sample. Can be measured as powder in air or helium or as pellet under vacuum.
Department of Ecosystem Science and Management
- Office 814-865-7541
- Fax 814-865-3725
Department of Ecosystem Science and Management
- Office 814-865-7541
- Fax 814-865-3725

